The #ImpatientRevolution eBook
This eBook is a guide for impatient patient organizations – those wanting to accelerate the development of new medicines for their disease by taking an active role. It explains why and how companies develop drugs for rare diseases, and how patient organizations can use this knowledge to analyze their disease field, to identify the main gaps, and to design strategies to get pharmaceutical companies to develop new treatments for their disease.
“#ImpatientRevolution is a free digital download eBook which is a must-read for charities about to embark on the journey towards drug development and clinical trials. Packed full of practical advice and tools, it’s a useful reference to guide you through the process.”
Summaries from conferences and notes on drug discovery and patient advocacy
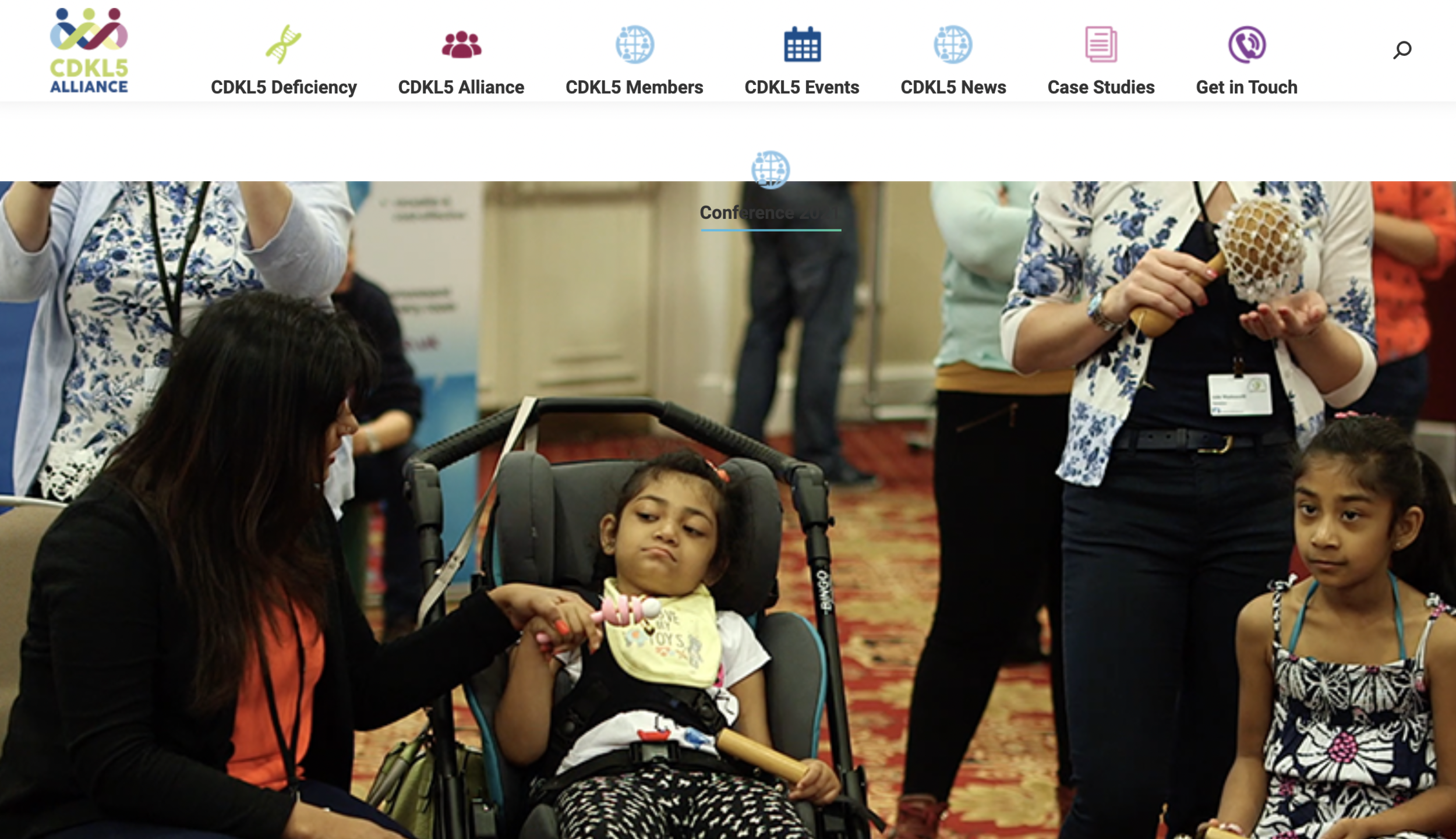
For the past ten years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2024 CDKL5 Forum that took place in October 28-29 in Boston
La décima edición del Foro CDKL5 tuvo lugar en Boston, los días 28 y 29 de octubre de 2024. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2024.
Patients with Dravet syndrome caused by missense mutations in SCN1A are candidates for the clinical trials with ASOs and gene therapies to increase SCN1A expression, and will be candidate for this therapies after approval.
These are my main learnings from the Syngap Conference 2023 celebrated in Orlando the day before the American Epilepsy Society meeting.
La novena edición del Foro CDKL5 tuvo lugar en Boston, los días 6 y 7 de noviembre. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2023.
For the past nine years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2023 CDKL5 Forum that took place in November 6-7 2023
Una conversación que suele salir mucho en reuniones de familias CDKL5 es la posición de la mutación de sus hijos en el gen, por si eso nos ayuda a saber la severidad de la enfermedad cuando crezcan. Os resumo abajo un listado de publicaciones y sus conclusiones.
The International Epilepsy Congress (IEC) is one of the largest epilepsy meetings attracting clinicians, researchers and the pharmaceutical industry. In 2023 it took place in Dublin, in early September. I review the progresses towards treating the cause of the rare epilepsies / DEEs
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. I review the progresses towards treating the cause of the rare epilepsies / DEEs.
For the past eight years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2022 CDKL5 Forum that took place in November 7-8 2022.
La octava edición del Foro CDKL5 tuvo lugar en Boston, los días 7 y 8 de noviembre. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2022.
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. These are my top 5 insights from the American Epilepsy Society 2021 meeting.
For the past seven years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2021 CDKL5 Forum that took place in November 1-2 2021.
CDKL5 deficiency is biologically reversible, bringing much hope and creating a sense of urgency for the development of restorative treatments for CDKL5 deficiency disorder (CDD). These are the conclusions of a publication from Zhaoland (Joe) Zhou’s lab from the University of Pennsylvania. The study was just published in the Journal of Clinical Investigation, and it is poised to become a landmark study in the field.
Last weekend, the CDKL5 deficiency disorder (CDD) community gathered in front of their computers for a virtual annual meeting organized by the International CDKL5 Alliance. For the 2021 Alliance meeting, the organizing team commission a series of pre-recorded videos to a large range of speakers and published the videos with subtitles so that all CDD families from all around the world could watch them at the same time. You can also access the videos at the CDKL5 Alliance website. Here is a personal summary of the 2021 CDKL5 Alliance meeting.
As of end 2020, the Dravet syndrome pipeline comprises 3 approved drugs, 11 drug candidates, and 12 different products have received orphan drug designations. Since the last report the pipeline has changed by, among others, the approval by FDA and EMA of Epidyolex (cannabidiol) and Fintepla (fenfluramine), the initiation of the first clinical trial with an antisense therapy, and progresses in different disease-targeting modalities including gene therapy approaches.
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. This year the meeting was virtual, which made the exhibit hall and the poster sessions less exciting but also made the presentations more accessible, including a 90-day on-demand period after the meeting to catch up with all of the parallel tracks. These are my own 5 insights from the American Epilepsy Society 2020 meeting.
For the past six years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the fourth Forum I attended, and my third since joining the Loulou Foundation.
Here are the main news and take-home messages from the 2020 CDKL5 Forum that took place in October 12-14 2020.
La sexta edición del Foro CDKL5 tuvo lugar online, los días 12-14 de octubre. El Foro es una reunión anual exclusivamente por invitación que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo. Este ha sido mi cuarto Foro CDKL5, y el tercero desde que me uní a la Fundación Loulou.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2020. [SPANISH VERSION - ALSO AVAILABLE IN ENGLISH]
There are multiple gene therapy and oligonucleotide programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy. Clinical trials have already started, with Stoke Therapeutics initiating the first clinical trial with a disease-targeting therapy in Dravet syndrome in summer 2020. Behind Stoke, gene therapies are approaching the clinic with Encoded Therapeutics having the most advanced clinical candidate and preparing for trials in 2021.
The rare genetic disease CDKL5 Deficiency Disorder (CDD) has been designated with a new disease code in the International Classification of Diseases (ICD), the medical classification list from the World Health Organization (WHO). The CDKL5 Deficiency Disorder diagnostic code will be incorporated in the October 1, 2020 classification revision.
This article covers what is an ICD-10, why it is important, and how patient communities can apply to have a code created for their disease.
Cada vez que se anuncia un nuevo ensayo clínico los pacientes y sus familias experimentan una mezcla de emociones. De un lado la alegría y esperanza de saber que vienen nuevos fármacos para su enfermedad. Del otro lado, muchas veces, desilusión y sensación de injusticia al ver que muy posiblemente no tengan la oportunidad de participar en el ensayo. Como científico involucrado en el diseño y ejecución de ensayos clínicos quiero compartir con los pacientes y sus familias algunas notas sobre por qué no todos los pacientes pueden entrar en un ensayo clínico, y si esto es “justo” o “injusto”.
El congreso de la Sociedad Americana de Epilepsia (AES por sus siglas en ingés) es la mayor cita en epilepsia del año, y como tiene lugar cada mes de Diciembre sirve también para hacer un repaso de como ha avanzado el campo a lo largo del ultimo año. AES 2019 fue el año de las terapias genéticas para las encefalopatías epilépticas y de desarrollo. Este artículo es el resumen de lo que me pareció mas interesante del congreso AES 2019
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. AES 2019 was the year of genetic therapies for the developmental and epileptic encephalopathies. This article highlights what I found the most interesting at the AES 2019 meeting.
La quinta edición del Foro CDKL5 tuvo lugar en Boston, los días 4 y 5 de noviembre. El Foro es una reunión anual exclusivamente por invitación que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo. Este ha sido mi tercer Foro CDKL5, y el segundo desde que me uní a la Fundación Loulou.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2019. [SPANISH VERSION - ALSO AVAILABLE IN ENGLISH]
For the past five years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the third Forum I attended, and my second since joining the Loulou Foundation.
Here are the main news and take-home messages from the 2019 CDKL5 Forum that took place in Boston in November 4 and 5.
The 2019 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 12 drug candidates in development for the treatment of Dravet syndrome, including 11 products that have received orphan drug designations. The Report includes the most recent updates on programs from GW Pharmaceuticals (Epidiolex / Epidyolex), Zogenix (Fintepla, ZX008), Biocodex (stiripentol) , Ovid Therapeutics (Soticlestat, OV935, TAK-935), Takeda Pharmaceutical, Supernus Pharmaceuticals (SPN-817, Huperzine), Xeris Pharmaceutical (diazepam), Epygenix Therapeutics (EPK-100, -200 and -300), NeuroCycle Therapeutics (NCT10015), PTC Therapeutics (ataluren), Stoke Therapeutics (STK-001), Encoded Therapeutics and OPKO Health (OPK88001, CUR-1915).
I used to say that at the patient communities “we set the agenda”. It turns out we didn’t, we were borrowing the agenda from scientific meetings. The 2019 CDKL5 Alliance International Research and Family Conference redefined what a patient-centered conference truly is. In this article I summarise the elements that make a meeting truly patient-centered.
There are currently 4 clinical trials ongoing or about to start in CDKL5 Deficiency Disorder: ataluren, ganaxolone, TAK-935 and fenfluramine. This article is a summary of where we are with clinical trials for CDKL5 Deficiency Disorder for families and other interested readers including what we know about these four drugs, their efficacy, at which level of clinical development they are at, and where can you learn more about these trials.
Many orphan drugs are advanced therapies. Pricing and access are major issues. Epilepsy is catching up with gene therapy. We shouldn’t call them rare diseases, but frequently misdiagnosed diseases. Either we wait 2,000 years for treatments or we start thinking “many diseases at a time”, and online patient communities are now part of the drug development process. That’s the short summary of the main lessons I took home from attending the World Orphan Drug Congress at the National Harbor April 10-12. The WODC one of the largest meetings dedicated to the development of new medicines for rare diseases and takes place once in the US and once in Europe every year. In a bit more detail, here is the expanded list of what I would like to share with you from the conference.
There are multiple gene therapy programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy. Clinical trials are around the corner, with Stoke Therapeutics expecting to initiate clinical trials in 2020. Just Stoke is not enough. New corporate players, and ideally some precompetitive collaboration around the common challenges of validating clinical outcome measures and biomarkers, are needed to maximize the success of gene therapies for Dravet syndrome.
2019 will be the year when we might have the European launch of Epidiolex, the US approval and launch of Fintepla, an ongoing clinical trial with TAK-935, hopefully some news about the ability of Translarna to improve Dravet syndrome by rescuing some of the nonsense mutations, and a year to prepare for the clinical trials that starting in 2020 will dominate the field: gene therapy approaches for Dravet syndrome that will treat more than just seizures. This entry reviews when we expect the main news about the Dravet syndrome pipeline during 2019.
2018 saw a record year in orphan drug approvals in Europe, but there are reasons to worry. Year after year, the number of orphan drug approvals in Europe is only one fifth to one third of the number of drug approvals in the US. Also, if orphan designations represent an early marker of the orphan drug development trend, then we might expect a decrease in the number of approvals in the immediate future. This article reviews the number of orphan drug designations and approvals in Europe in the 2000-2018 period to understand the trends that might impact the number of orphan drug approvals in the next few years.
With 2018 now behind us, it is time to review how well companies working on Dravet syndrome delivered based on the timelines that they had announced at the beginning of the year. While it is hard to predict exactly when many milestones are going to happen, in particular those still over half a year away or more, the class of 2018 did quite well overall, and many of the news that we were expecting took place on schedule – with some exceptions.
The Food and Drug Administration (FDA) set a new record in 2018 with the highest number of new drug approvals in the last two decades. The FDA also set a new record in orphan drug approvals in 2018, granting 86 new marketing authorizations for drugs treating rare diseases. In this article I review the delay between orphan drug designation and orphan drug approval, and identify how in many cases orphan drugs wait 10 or more years after reviewing the orphan drug designation and before they get approved.
Every year the American Epilepsy Society (AES) meeting gets larger. This year, over 6,000 people gathered in New Orleans to discuss the latest information about epilepsy care and the development of new treatments for epilepsy. The 2018 meeting captured the latest developments in the field of epilepsy drug development, where rare disease populations and new technologies are two areas of considerable growth and that are changing the way we will treat epilepsy. This article highlights what I found the most interesting at the AES 2018 meeting.
When you let the scientific community know that your organization is open to fund research around your rare disease you are likely to get many research proposals from academic groups. These will come in different qualities, and will have different relevancefor your disease. This entry adds to Impatient Series I and to the ImpatientRevolution book and discusses in more detail how to determine that a proposed project is the right one and how to monitor its progress.
For the past four years the Loulou Foundation hosts an annual “by invitation only” meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the second Forum I attended, and my first since joining the Loulou Foundation.
Here are the main news and take-home messages from the 2018 CDKL5 Forum that took place in London, UK, in October 22 and 23.
Once there is a drug that has been developed, it is very clear why talking with patients and collaborating with them is useful for pharmaceutical companies. What is less obvious to both companies and patient organizations is how patients can be active in research before there is any drug in development, during the preclinical phase, and how this might lead to medicines being developed. Some patient organizations might start by funding some academic group, but without a clear vision and a longer-term strategy the return on those efforts will be compromised, and medicines will take longer to come. Here I want to outline the broader strategy that patient organizations can follow to advance research towards new medicines even before companies are working on it.
In the previous entry we discussed the many types of companies that get interested in rare diseases, each of them because of different reasons. If your disease field matches one of those business propositions you will get at the top of the list of interesting diseases for that company, but another part of the decision equation is time, or field maturity. Is this field ready for us to start working on it already The following are the questions that a company will often need to answer to be able to judge if the field is ready for them, too early for them, or maybe even too mature for them to get in.
The number of orphan products in development keeps growing every year. A particular rare disease will be attractive for a drug development company if it matches one of four main business propositions determined by the interplay of market pressure and technology enablers. The degree of maturity of a field will also attract companies and help prioritize which disease to focus on where multiple rare diseases match the needs of the company or the drug that they are already developing
Most patient organizations, no matter how small, start with the same mission: to raise awareness and funds for research in their disease. But what research are they doing? are they all starting in the same place? and do they all evolve to run the same type of research? In my experience there are many types of strategy and approaches that patient groups take, in particular when they are starting. What a patient organization means when they say “we are doing research”, therefore, hides many different possibilities. The article describes some of these types, and discusses their advantages and disadvantages.
INTRODUCTION TO THE SERIES - The field of rare diseases, where each disease affects only hundreds or thousands of patients world-wide, is leading this revolution. Their diseases were once orphan to medicine, too rare to receive sufficient attention and investment to move the needle. Today patients take ownership of their research fields, and move the needle themselves. They have become the center of the network and the expert in the room. They are impatient, not wanting to wait for pharma to take an interest in their disease. They are impatient patients and are leading an impatient revolution.
Every other year the International League Against Epilepsy organises a major epilepsy medical congress in Europe called the European Congress on Epilepsy (ECE). This year I attended the main three days of the ECE addition in Vienna, looking at the field partly as a drug developer and partly as a patient advocate working on behalf of rare epilepsy patient communities. Here is the list of what I found the most interesting at the ECE 2018 meeting.
Dravet syndrome pipeline review 2018 - Summary in Spanish for Dravet families.
Los avances en torno al tratamiento del síndrome de Dravet en los últimos años han seguido pasos agigantados. En los últimos 5 años hemos experimentado una explosión en el número de programas en desarrollo para tratar la enfermedad: de tener solo Diacomit y nada más a tener Diacomit y 14 más en desarrollo. Este texto es un resumen en Español del Dravet syndrome pipeline review 2018 dirigido a familias, con el texto no solo traducido sino también ajustado a los intereses de aquellos que tienen un hijo/a con síndrome de Dravet y su entorno.
The 2018 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 14 drug candidates in development for the treatment of Dravet syndrome, including 9 products that have received orphan drug designations. It also includes an analysis of the competitive landscape and evaluates current and future opportunities of the Dravet syndrome market.
After May 2018 patients will have the right to request access to their data files. They will have the right to withdraw their data from a database that is not being used in the way that the patient through it would operate. And they will have the right to obtain these files generated by a particular data holder, for example a gene testing lab, and get the usable file and share it with as many studies and registries as the patient wants. The EU General Data Protection Regulation gives patients the Right to Access, the Right to be Forgotten, and the Data Portability right.
There is no doubt that AAVs will support the development of many gene therapies for genetic diseases, but for many diseases AAVs are too small to carry a copy of the gene that patients need. There is a strong need to find non-AAV alternatives that can provide a suitable gene therapy option for those diseases caused by mutations in large genes. Within Dravet syndrome, these next-generation therapies are being led not by companies but by academic groups with the support of patient organizations. This is a review of these programs and how they are attempting to develop a gene therapy for treating Dravet syndrome.
we expect in 2018 the first approval of a treatment for Dravet syndrome in the US, the results of clinical trials with three new therapeutics (ZX008, ataluren and OV935/TAK935), and the initiation of the first clinical trial ever done with a disease-modifying therapy designed to treat Dravet syndrome (OPK88001). 2018 is going to be a good year.
Every year the American Epilepsy Society (AES) meeting gets larger. This year AES meeting was very positive, showing a great progression of the field that now moves towards orphan indications (with new drugs and clinical designs), where patient organizations gain relevance (also a characteristic of the orphan drug field), and where we start having new drugs able to break the barrier of pharmacoresistance.
Should we talk about syndromes based on the gene that causes them or should we talk about them (and treat them) based on the clinical characteristics that they display? Earlier this month, the epilepsy community gathered in Barcelona for the 32nd International Epilepsy Congress and there was a debate between genetic and symptom-base syndrome classification. This debate goes beyond semantics, and has important regulatory and access implications.
June 23 is a special day for families of people with Dravet syndrome. It is the International Dravet Syndrome Awareness Day, that in 2017 celebrates its 4th edition. That's why today we announce the publication of the 2017 Dravet Syndrome Pipeline and Opportunities Review, a market research publication that provides an overview of the global therapeutic landscape of Dravet syndrome.
Gene therapy is here, VCs want to invest in orphan drugs, Rettsyndrome.org foundation is doing a great work, the line between patients and drug developers is blurring, we should start talking about industry (not patient) engagement, networking is not always easy, and Fulcrum Therapeutics should be in your list of companies to watch.
In January of 2017, the European Medicines Agency approved everolimus for the treatment of seizures in tuberous sclerosis complex, becoming the first anti-epileptic medication ever approved. But there are more than 25 different molecular entities approved as anti-epileptic medications, so let me explain you why this case is different and why it represents a big milestone for the epilepsy field.
We are one week away from the Rare Disease Day and the global theme this year is Research. To celebrate this day and honor the theme I am releasing the eBook #ImpatientRevolution, a guide for impatient patient organizations.
February 28 is the Rare Disease Day, and the global theme this year is research.
People from all over the world will come together this month to advocate for more research on rare diseases, and to recognize the critical role that patient organizations play in research.
I am excited to join this year Rare Disease Day and announce the launch of my first eBook: #ImpatientRevolution, a guide for impatient patient organizations.
With 2016 numbers now available, the number of orphan drugs in development for neurological indications is looking quite positive. I have reviewed the numbers of orphan drug designations and approvals by FDA in 2016 to see how popular are neurological orphan drugs today and what the trend is for the near future.
There was one clear star at the American Epilepsy Society Annual meeting this year, Epidiolex (cannabidiol). Yet four years ago no one would have thought that the cannabis-derived drug would take over the field of epilepsy in such a big way.
The journal Nature just released one of the most anticipated breaking news of the last few years: CRISPR gene editing has been tested in a person for the first time. In my day-to-day work I interact with families that have a child with a genetic disease. I get one question a lot: how close are we to turn that discovery into a therapy for people with genetic diseases?
When a doctor prescribes you a medication you will be in one out of four groups of patients: 1) you might have experience and no side effects, 2) you might experience efficacy with side effects, 3) you might not have efficacy nor side effects, or ...
One day I sent an e-mail that changed my life. During my training as a scientist, I had learned how to study what goes wrong with the brain and to research how we could fix it. Then one day I sent an e-mail to a patient.






























































The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. These are my main insights from the American Epilepsy Society 2024 meeting.